Abstract
Background: Several clinical and genetic factors negatively impact on survival in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), including TP53 allelic state, presence of a complex karyotype (CK) and blast count. Moreover, MDS with biallelic TP53 inactivation has recently been classified as separate entity according to the WHO classification 2022. However, questions remain regarding the influence of TP53 alterations (alt) and CK dependent on the number of blasts in MDS and the interplay of TP53alt and CK in AML.
Aim: (1) Analysis of the frequency of TP53 single hit (sh: mutations (mut), deletions (del), cnLOH) and double hit (dh: mut+del, ≥2 mut or mut+cnLOH) events in MDS with <5% blasts, ≥5%,<10% blasts, ≥10%,<20% blasts and in AML. (2) Determination of overall survival (OS) dependent on the TP53 allelic state in the subgroups. (3) Univariate and multivariate analysis of TP53 state, CK and blast count.
Methods: Whole-genome sequencing (WGS) was performed for 773 AML and 747 MDS patients (median coverage 100x). MDS cases were subdivided into <5% blasts (n=419), ≥5%,<10% blasts (n=175) and ≥10%,<20% blasts (n=153). 151bp paired-end reads were generated on NovaSeq 6000 and HiSeqX machines (Illumina, San Diego, CA). SPSS (version 19.0.0) software (IBM Corporation, Armonk, NY) was used for statistical and for OS analysis (Kaplan-Meier). All reported p-values are two-sided and were considered significant at p<0.05.
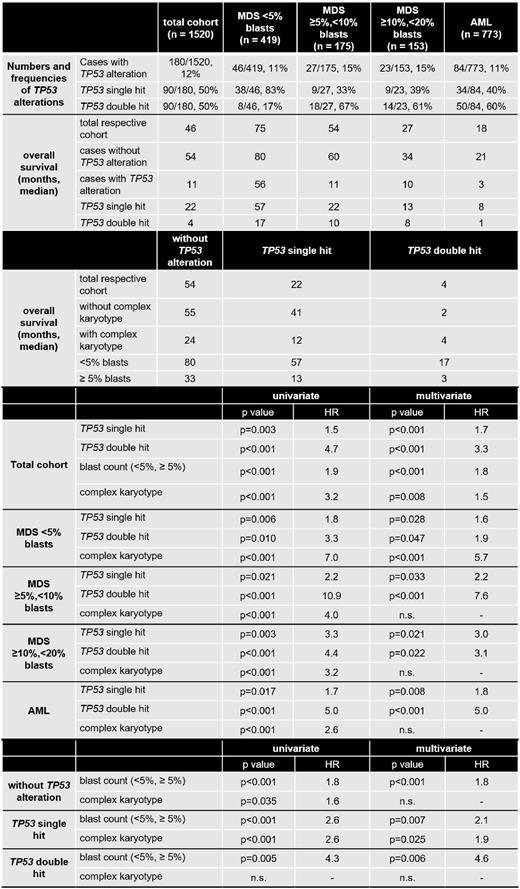
Results: The frequency of TP53alt was comparable between MDS <5% blasts (46/419 cases with TP53alt, 11%), MDS ≥5%,<10% blasts (27/175, 15%), MDS ≥10%,<20% blasts (23/153, 15%) and AML (84/773, 11%). However, MDS cases with <5% blasts clearly differed from the other subgroups by showing predominantly TP53sh events (83% of cases with TP53alt), whereas in the other subgroups a TP53dh was detected at high frequencies (in 67%, 61% and 60% of cases with TP53alt, respectively). Regarding OS in the subgroups, MDS <5% blasts showed the best outcome (median OS, 75 months), followed by MDS ≥5%,<10% blasts (54 months), MDS ≥10%,<20% blasts (27 months) and AML (18 months). The same hierarchy was observed when each subgroup was divided into cases with and without TP53alt, cases with TP53alt showing a worse prognosis (56, 11, 10 and 3 months, respectively) than cases without TP53alt in each subgroup (80, 60, 34 and 21 months, respectively). Further subdivision of TP53alt into TP53sh and dh revealed a dismal outcome of TP53dh in all subgroups, again following the hierarchy described above (TP53dh: 17, 10, 8 and 1 month, respectively). In univariate Cox regression analysis, TP53sh (hazard ratio (HR)=1.5, p=0.003), TP53 dh (HR=4.7, p<0.001), blast count (<5% vs. ≥5%; HR=1.9, p<0.001) and CK (HR=3.2, p<0.001) had an adverse impact on OS in the total cohort. Multivariate Cox analysis including these parameters revealed an independent adverse impact on OS for all factors, with TP53dh being the strongest prognostic factor. When univariate Cox analysis were performed for the respective subgroups (MDS <5% blasts; ≥5%,<10%; ≥10%,<20%; AML) using TP53sh, TP53dh and CK as parameters, all factors showed a negative influence on OS in all subgroups. However, in multivariate analysis, an independent adverse impact on OS regarding CK was found only in cases with MDS <5% blasts (HR=5.7, p<0.001), whereas TP53sh and dh were independent factors in all subgroups, TP53dh showing the strongest influence. When the total cohort of 1520 cases was split into cases without TP53alt (n=1340), with TP53sh (n=90) and dh (n=90), only the blast count had an independent adverse impact on OS in multivariate analysis in all subgroups (HR=1.8, p<0.001; HR=2.1, p=0.007; HR=4.6, p=0.006, respectively), whereas the presence of a CK showed a negative impact in cases with TP53sh only (Table).
Conclusions: (1) MDS cases <5% blasts separate from MDS cases ≥5% blasts and from AML cases by the predominance of TP53sh and by being the only subgroup in which a CK showed an independent adverse impact on OS. Thus, the presence of a CK generally plays a minor prognostic role. (2) The remaining subgroups (MDS ≥5%,<10% blasts; ≥10%,<20% blasts; AML) display a number of similarities, as TP53dh is found at high frequencies and only TP53alt but not a CK independently influences OS. (3) In the total cohort, TP53dh is the strongest prognostic factor; however, also the blast count influences OS independent of TP53 allelic state.
Disclosures
Stengel:MLL Munich Leukemia Laboratory: Current Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Walter:MLL Munich Leukemia Laboratory: Current Employment. Baer:MLL Munich Leukemia Laboratory: Current Employment. Nadarajah:MLL Munich Leukemia Laboratory: Current Employment. Hutter:MLL Munich Leukemia Laboratory: Current Employment. Kern:MLL Munich Leukemia Laboratory: Current Employment, Other: Ownership. Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal